Published online 2018 Aug 29
Authors: Christopher M. Proctor, Andrea Slézia, Attila Kaszas, Antoine Ghestem, Isabel del Agua, Anna-Maria Pappa, Christophe Bernard, Adam Williamson, and George G. Malliaras
The persistence of intractable neurological disorders necessitates novel therapeutic solutions. We demonstrate the utility of direct in situ electrophoretic drug delivery to treat neurological disorders. We present a neural probe incorporating a microfluidic ion pump (μFIP) for on-demand drug delivery and electrodes for recording local neural activity. The μFIP works by electrophoretically pumping ions across an ion exchange membrane and thereby delivers only the drug of interest and not the solvent. This “dry” delivery enables precise drug release into the brain region with negligible local pressure increase. The therapeutic potential of the μFIP probe is tested in a rodent model of epilepsy. The μFIP probe can detect pathological activity and then intervene to stop seizures by delivering inhibitory neurotransmitters directly to the seizure source. We anticipate that further tailored engineering of the μFIP platform will enable additional applications in neural interfacing and the treatment of neurological disorders.
The failure of systemic drug treatments to address numerous neurological disorders has spurred the development of alternative approaches that attempt localized treatment. These localized treatments focus therapy on the region of the brain affected by the pathology, thereby enhancing the effectives of the treatment while reducing side effects inherent in systemic treatments. Some of these approaches such as optogenetics (1–4) and designer receptors exclusively activated by designer drugs (5) have shown promise but have been limited by safety concerns about the need for viral transfer of engineered proteins and molecules (6). Other treatments such as syringe injection or convection-enhanced delivery of drugs are prone to clogging or reflux and may also cause local edemas due to the pressure increase at the injection point (7–9).
In this work, we show that electrophoretic drug delivery can overcome the above problems by coupling the benefits of a highly localized, on-demand treatment with drugs that specifically control cellular functions. We demonstrate the therapeutic potential using epilepsy as a model system. Epilepsy, which affects 1% of the world population and remains drug-resistant in 30% of the cases (10, 11), is a prototypical example for which systemic drugs have failed in the clinic despite having strong antiepileptic effects because of their side effects, toxicity, and/or failure to cross the blood-brain barrier (12). The intermittent nature of epileptic seizures makes the disorder particularly well suited for a therapeutic approach such as electrophoretic drug delivery that acts only when needed, for instance, just before the onset of a seizure.
Here, we present an electrophoretic drug delivery device based on the organic electronic ion pump (OEIP), which offers the ability to deliver drugs with precise spatiotemporal control (13, 14). In contrast to other drug delivery devices, the ion pump works by electrophoretically pumping ions across an ion exchange membrane and thereby delivers only the drug of interest and not the solvent (aside from a few water molecules per ion that make up the hydration shell). This “dry” delivery is of paramount importance for biological interfacing as it enables an intimate interface between the drug delivery outlet and the target cells, allowing large amounts of drug delivery with negligible local pressure increase. Previous reports of OEIPs have shown much promise for interfacing with biology; however, challenges in scaling down these devices have limited their in vivo applications within the brain to date
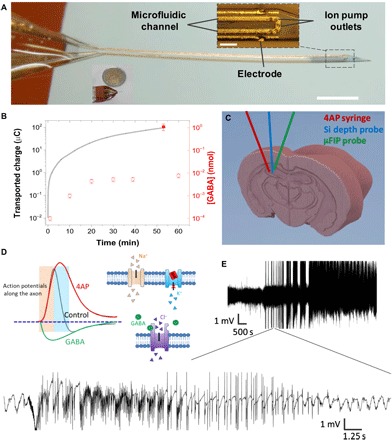
Looking ahead, we believe that electrophoretic drug delivery devices can be further adapted to best treat epilepsy and other neurological disorders. While epilepsy is particularly challenging as it presents in many forms, it has been found that approximately 60% of the patients have a single focal point (45), and these are the patients that are most likely to find conventional drug treatments inadequate (46). Thus, a single μFIP implant at the seizure focus may prove a viable treatment option for these patients. On the other hand, patients with nonfocal epilepsy may benefit from the use of multiple μFIP implants and/or a single implant with multiple drug delivery outlet locations. In addition to epilepsy, we foresee that a μFIP implant could be used, for instance, to deliver dopamine for the treatment of Parkinson’s disease with the possibility of using integrated recording sites to optimize the dosing frequency. Likewise, a μFIP implant could deliver chemotherapeutic agents to an inoperable brain tumor and/or to the tissue surrounding a resected tumor. These topics will be the subject of future research.
Commentary
Citation
Proctor, C. M., Slézia, A., Kaszas, A., Ghestem, A., del Agua, I., Pappa, A.-M., Bernard, C., Williamson, A., & Malliaras, G. G. (2018). Electrophoretic drug delivery for seizure control. Science Advances, 4(8). https://doi.org/10.1126/sciadv.aau1291