By: Jeffrey K Aronson
Published in the Journal of Expert Opinion on Drug Safety
03 Mar 2005
If drug names are similar, errors can occur. Problems arise when different drugs have similar names (whether proprietary or non-proprietary), when formulations with the same brand name contain different drugs, when the same drug is marketed in formulations with different names, and when drug names are abbreviated. The risk of errors could be reduced by some simple precautions at different stages of drug development, prescribing, supply, and administration. Regulatory authorities and manufacturers should maintain their vigilance when naming new drugs and formulations
and should be prepared to change names if errors occur. Before they write an unfamiliar name on a prescription, prescribers should check what they are prescribing and what other medications the patient is taking (patients should be familiar with their medicines), and pharmacists should check patients’ medicines. At all times there should be good communication among those who prescribe, supply, and administer medicines, and those
who take them.
The naming of drugs
‘Dear Doctor, Mr Smith has been taking chlorampicillin for some time. I think that
he could now stop.’ Chlorampicillin doesn’t exist; the patient was taking chlorambucil. Prescribers can just as easily confuse real drug names, many of which look and
sound and alike, as this doctor confused the name of a real drug with that of an
imaginary one.
Drugs usually have three different names:
• A chemical name, the form of which generally follows the rules issued by the
International Union of Pure and Applied Chemistry (IUPAC).
• A non-proprietary name. This is usually the International Non-proprietary Name
(INN), either recommended (rINN) or proposed (pINN) by the WHO (World Health Organization). However, it may be a locally approved name – for example,
the British Approved Name (BAN) or United States Adopted Name (USAN).
• A proprietary name (brand name or trade name), assigned by a pharmaceutical manufacturer.
For example:
• Chemical name: (R)-1-(3,4-dihydroxyphenyl)-2-methylaminoethanol.
• INN: epinephrine.
• BAN: adrenaline.
• Proprietary names (UK): Anapen, Epipen.
Some drugs have no non-proprietary names other than the chemical name (e.g., glyceryl trinitrate, acetylsalicyclic acid). In this report, upper case initials are used for brand names and lower case initials are used for non-proprietary names.
Sources of confusion
The possible sources of confusion over drug names [3,4] can be considered under four headings:
• Different drugs with similar names.
• Formulations with the same brand name containing different drugs.
• The same drug marketed in formulations with different names.
• Abbreviated drug names.
Avoiding errors – Prescription writing
A doctor told a 55-year-old man to use Remotic (triamcinolone and halquinol) eardrops. Instead, the patient swallowed the Rivotril (clonazepam) tablets that his chemist
dispensed and became unfit to drive. Another doctor commented, ‘That a supposedly intelligent patient, having been told to use capsules of Remotic in the ear, should then eat tablets of Rivotril will surprise no one with 30 years in general practice’ [39]. That a doctor’s handwriting may be so bad is equally unsurprising [40].
A psychiatrist prescribed Concordin (protriptyline). The pharmacist dispensed Coumadin (warfarin). The patient suffered multiple haemorrhages [41]. In a similar case, procyclidine (Kemadrin) was confused with Coumadin [42]. Careful prescription writing is important. Increasingly, prescriptions are being computer-printed; the use of a large, bold font will help pharmacists to avoid misreadings. Hand-written prescriptions should be penned carefully, with drug names in block capitals. In one case, illegible handwriting led to the dispensing of lorazepam instead of Magnapen, and the patient crashed his car; in another case, Daonil (glibenclamide) was dispensed instead of Amoxil, and the patient suffered irreversible brain damage [15]. Other errors have involved hydroxyzine/hydralazine and DesOwen (desonide) ointment/ Dovonex (calcipotriene) ointment [40]. If pharmacists have doubts about poorly legible drug names they should contact the prescriber. In hospitals, clinical pharmacists should check prescriptions and liaise with prescribers. Prescribing by non-proprietary names probably reduces the
risk of confusion. Most of the reported examples of confusion have been with proprietary names and there are more proprietary than non-proprietary names in the confusable pairs listed in comprehensive tables. Abbreviations of drug names should never be used.
Avoiding errors – Communication
Informing the patient about drug therapy and its possible adverse consequences should help in avoiding errors of confusion. This includes giving patients information leaflets and
carefully labeling containers. When patients were given written information about their drug therapy upon leaving the hospital, 86% of them knew the names of their drugs 12 weeks later, compared with 47% of those who had not been given information [43]. Good communication between different prescribers and with pharmacists also helps. Patients should be encouraged to bring their medications with them when consulting a prescriber, to enable errors to be detected [44]. Pharmacists are well aware of the problems and generally take care to avoid them. They should ask patients to return old medicine containers, in order to identify previous therapy and check that patients know what therapy they are currently taking. They should be especially careful with sulfonylureas, which have often been implicated in medication errors, and keep them in a separate section in the dispensary, in order to highlight the problem. These and other recommendations are summarised in Table 2.
Source:https://www.tandfonline.com/doi/pdf/10.1517/14740338.3.3.167
Image: www.devinemedical.com
Analysis:
This article was selected because it shows that within the community and world we have constructed many things can cause a person to lack pharmaceutical literacy. Even when all of the correct measures have been taken, the prescription itself can be wrong and thus create health issues later on due to an error in prescription. I found it fascinating that when the patients were given leaflets/written information about their drug, “86% of them knew the names of their drugs 12 weeks later.”
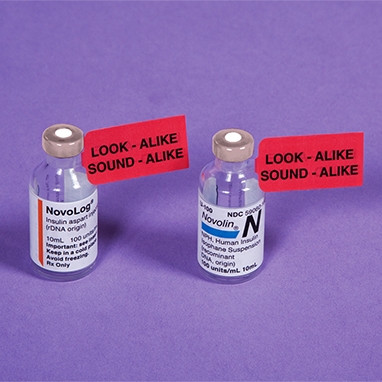



This was a great article that addresses the ways in which medications are not successful in comprehension with its corresponding patients. As a result, there is a potential and immediate effect that can inhibit an individual and their ability function “normally”, for example ability to drive. Understanding where individuals go wrong and target ways in which one can confidently identify the correct medication, of the right brand, of the right dosing etc. is a priority.